905
Views & Citations10
Likes & Shares
Diabetes is a
chronic metabolic disorder that affects a large number of populations globally.
Approximately one-fourth of people suffering from diabetes will develop an
ulcer over the foot (Diabetic Foot Ulcer- DFU) during their lifetime. Pus
samples were collected from the deep base of the ulcer using sterile swabs. The
organisms were identified by direct Gram staining, colony morphology and
biochemical reactions. Antibiotic susceptibility testing was performed using
the Kirby Bauer disk diffusion method according to the Clinical and Laboratory
Standards Institute (CLSI) guidelines. Total 98 patients with type 2 diabetes
(T2D) mellitus were included; the susceptibility to DFU is significantly more
common in the males. Patients with more than 60 years of age have a high
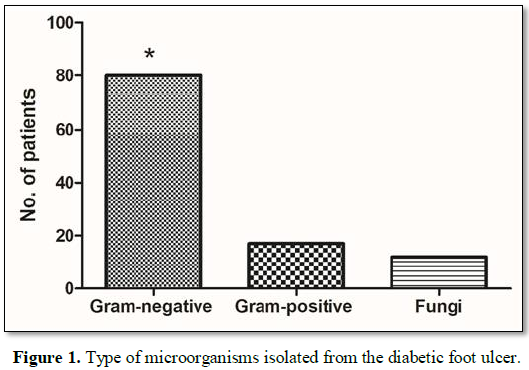
prevalence of DFU. Eighty patients had a gram -ve bacterial infection and 17
had gram +ve infections. Fungal infection in 12 (Candida non-albicans in 8, Candida albicans in 2 and Mucomycosis in
2). The gram -ve bacterial infections were significantly higher as compared to
other microorganisms. Among the gram-positive infections Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus (MRSA) infection
was common. Gram-negative bacteria showed maximum sensitivity to Amikacin in 51
(63.7%), Meropenem in 45 (65.2%) and Imipenem in 44 (72.1%) patients.
Gram-positive bacteria showed sensitivity to Teicoplanin in 5 (83.3%) patients
and Vancomycin in 4 (80%). DFU causing fungus, Candida non-albicans showed
sensitivity to Amikacin in 7 (87.5%), Meropenem in 6 (75%), Imipenem in 7 (87.5%);
Candida albicans showed max.
sensitivity to Ceftriaxone (100%); Mucomycosis showed sensitivity to Amikacin
and Meropenem (each 100%). DFU were predominantly due to Gram-negative
bacteria, such as Escherichia coli, Pseudomonas spp. and Klebsiella oxytoca. Amikacin,
Ciprofloxacin, Meropenem, Imipenem and Ceftriaxone were most sensitive
antibiotics.
INTRODUCTION
Diabetes
Mellitus is a chronic metabolic disorder that affects a large number of
populations globally and is a major public health problem [1-3]. Approximately
one-fourth of people with diabetes will develop an ulcer over the foot (Diabetic
Foot Ulcer- DFU) during their lifetime and as many as half of these ulcers will
become infected [4,5]. In the people with diabetes mellitus and foot ulcers,
several factors, such as inappropriate antibiotic treatment, the chronic nature
of the wound, and frequent hospital admission, can influence the presence of
multidrug-resistant microorganisms in the foot ulcer [6,7]. Moreover, the
specific organism identified in diabetic foot infections can differ not only from
patient to patient and hospital to hospital but also from one part of the
country to another [6,8,9].
The
WHO has projected that the maximum increase in diabetes mellitus will occur in
India [10]. India has nearly 33 million diabetic subjects today, which is
mainly from the urban population. The scenario is also rapidly changing in
rural areas. Diabetes India study confirms that the WHO estimate of 35
million adults with diabetes
in India today
Most
diabetic foot infections are real emergencies; therefore, antibiotic therapy
should be started immediately, to improve the chances of salvaging the limb.
Initial empirical therapy should be based on clinical presentation,
gram-staining results and knowledge of the organisms that are most frequently
isolated from a particular infection [9,14,15].
The
appropriate selection of antibiotics based on the antibiograms of isolates from
diabetic foot infections is extremely critical for the proper management of
these infections [14,16]. Therefore, the aim of the present study was to
evaluate the bacteriological profile of diabetic foot ulcers at our hospital,
in order to determine the relative frequencies of bacterial isolates cultured
from foot infections and to assess the in
vitro antibiotic resistance and susceptibility of the isolated bacteria to
a variety of commonly used antibiotics.
MATERIALS AND METHODS
Study design and patients
This
hospital-based retrospective study includes 98 patients (11 females) with
diabetic foot ulcers, who were admitted to the SGPGIMS, Lucknow, India. The
study was conducted over a period of 24 months. Demographic and lesion data,
including age, sex, duration of diabetic foot, diabetes medications used,
features of the lesion and location of the lesion, were recorded for each
patient.
Inclusion criteria: Foot ulcer patients
who diagnosed or suspected to have diabetes mellitus and confirmed by elevated
fasting as well as postprandial blood sugar.
Exclusion criteria: Healthy people who
were suspected with foot ulcer having normal fasting and post-prandial blood
sugar.
Sample processing
Samples
were collected deep from the base of the ulcer using two sterile swabs. One
swab was used for gram staining and the other was used for culture. To
eliminate the possibility of isolating colonizing bacteria, superficial ulcers
were excluded from the study. Direct gram-stained smears were examined under
the microscope to evaluate a relative number of microorganisms and their
morphological characteristics. Any fungal elements observed were confirmed by
KOH preparation. The samples for culture were inoculated onto 5% Sheep blood
agar (SBA), Chocolate agar and MacConkey’s agar medium and incubated at 37°C
for 24 h in 7-10% CO2 concentration and the plates were examined for
growth. Sabouraud’s dextrose agar slopes were used for culture of fungus. The
organisms were identified by direct Gram staining, colony morphology and biochemical
reactions.
Characterization of bacterial isolates
After
rinsing the wound area with saline and debriding the wound, swab/tissue samples
were collected aseptically from the wound, conditioned in Stuart medium and
immediately taken to the microbiology laboratory. The specimens were inoculated
on blood and MacConkey agar plates for the isolation of aerobic bacteria.
Additionally, thioglycolate broth and mannitol salt agar were inoculated. The
media plates and broth were then incubated at 37°C for 24 h. The isolates were
identified based on colony morphology, gram-staining results, motility, a
catalase test, an oxidase test, a coagulase test and biochemical tests.
Antibiotic susceptibility testing
Antibiotic
susceptibility testing was performed using the Kirby Bauer disk diffusion
method according to the Clinical and Laboratory Standards Institute (CLSI)
guideline [17]. The antibiotics tested for Gram-positive bacteria were
azithromycin, amoxicillin/clavulanic acid; cefoxitin, cefalexin/cefalotin,
erythromycin, imipenem, oxacillin, penicillin, trimethoprim-sulfamethoxazole
and vancomycin, while the antibiotics tested for Gram-negative bacteria were
amoxicillin/clavulanic acid, amoxicillin, ampicillin, aztreonam, cefotaxime,
cefoxitin, gentamicin, imipenem, polymyxin B, norfloxacin and tetracycline.
Using the broth macrodilution (tube) method (minimum inhibitory concentration
(MIC)), the modified Kirby-Bauer disk diffusion method was validated for
vancomycin and polymyxin B susceptibility testing of Staphylococcus aureus and Pseudomonas
spp., respectively. MICs were determined and interpreted according to the
criteria of the CLSI [17]. Staphylococcus
spp. were tested for methicillin resistance using oxacillin and cefoxitin disks
as recommended by the National Committee for Clinical Laboratory Standards and
according to the criteria of the CLSI, respectively. Novobiocin disks were used
to distinguish Staphylococcus
saprophyticus, which is resistant to novobiocin in culture, from other
coagulase-negative staphylococci (CONS). Streptococcus
pneumoniae isolates were identified based on standard laboratory procedures,
including colony morphology on blood agar and optochin sensitivity tests [7]. Streptococcus pyogenes isolates were
confirmed with blood agar culture and a bacitracin test, which is used in the
presumptive identification of group A, beta-hemolytic streptococci.
Multidrug-resistant organisms (MDROs) were defined as bacteria that were
resistant to more than one or all classes of antibiotic [17-21].
STATISTICAL ANALYSIS
The
statistical analysis was carried out using the SPSS software, version 23.0 and
Fisher's Exact Test was used to verify the association between antibiotic use
and Gram-negative bacteria resistance. In descriptive statistics, the frequency
of isolate distribution and antibiotic resistance was treated as categorical
variables. The chi-square or two-sided Fisher’s exact test was used to
discriminate whether the distributions were significantly different between
different groups. The distributed variables were expressed as the Mean ± SD and
compared by one-way ANOVA. Variables without a normal distribution were
expressed as the median (interquartile range) and compared by Kruskal-Wallis H
test. It was considered statistically significant if the two-side p-value is
less than 0.05.
RESULTS
We
enrolled 98 patients with diabetes and of these 11 were female. The
susceptibility to foot ulcer is significantly (p<0.01) more in male patients
than in female patients. The median age of the patients was 57.50 (23-60)
years. We also found that patients with more than 60 years of age have a high
prevalence of DFU.
Gram-negative bacteria showed sensitivity to
following antibiotics (Amikacin 51 (63.7%), Ceftazidime 27 (36%), Ceftriaxone
26 (45.6%), Ciprofloxacin 27 (47.4%), Meropenem 45 (65.2%) and Imipenem 44 (72.1%)).
Imipenem was the most effective antibiotic against Staphylococcus bacteria.
Gram-positive bacteria shows sensitivity to Teicoplanin in five (83.3%) patients
and Vancomycin in four (80%). Details of sensitivity were shown in Table 2.
DISCUSSION
DFU
is a global, complex and expensive health problem. The emergence of
antimicrobial resistance to selective drug limits the usage of antibiotics to
only clinically infected foot ulcers and to use the anticipated spectrum of
antimicrobial or else untreated DFU may risk for limb loss [9,22,23].
In
the present study, we found that elderly patients (˃60 years of age)
constituted the majority of patients with foot infections. This may be
explained by the fact that foot lesions occur commonly among patients with
long-standing diabetes mellitus, particularly the elderly and those with
sensory neuropathy [22]. Similar to the previous study we noted that the
susceptibility to foot infections is greater in male patients than in female
patients [24,25].
Diabetic
foot ulcers are colonized by pathogenic bacteria that may predispose a
susceptible patient to a lower extremity infection, defined as the invasion and
multiplication of microorganisms in body tissues associated with tissue
destruction or host inflammatory responses [26].
Our
studies have reported that Gram-negative bacteria were predominant. Aerobic
Gram-negative bacteria (mainly Enterobacteriaceae and sometimes Pseudomonas aeruginosa or other
Gram-negative species) are usually isolated in conjunction with Gram-positive
cocci in patients with chronic or previously treated infections.
The
prognosis of diabetic foot infections remains poor, and the outcomes have been
reported to be worse with MDROs than with non-MDROs in patients with diabetic
foot infections. Our study showed that MDROs were common in hospitalized
patients with chronic and acute wounds. An increase in the occurrence of
chronic wound infections with MDROs in the diabetes mellitus population has
been noted over the last decade and has been primarily attributed to MRSA, but
antibiotic-resistant Gram-negative organisms, particularly Pseudomonas aeruginosa, have also been implicated [27,28]. In our
study, few patients underwent some type of amputation. However, almost all
patients had chronic wounds caused by monomicrobial infections of Gram-negative
bacteria and polymicrobial infections. Moderate to severe infections often
necessitate empirical regimens with activity against commonly isolated
Gram-negative bacilli, MRSA and perhaps Enterococcus species [29]. Mild
infections are often managed with local wound care strategies and/or
prophylactic measures. It is important to note that the decisions relating to
the antibiotic treatment of wounds are influenced by clinical evidence, the
availability of appropriate antibiotic interventions, patient's requirement and
practitioner's expertise [30].
The
antibiogram-resistogram pattern study of gram-negative bacteria isolated from
DFU patients showed that Escherichia coli,
Klebsiella oxytoca and Pseudomonas
species are common. On the other hand, Gram-positive bacteria isolated from the
foot ulcers of patients with diabetes showed that Staphylococcus aureus was the predominant pathogen.
Enterobacter spp. was resistant to the majority of
antibiotics tested, which is consistent with the findings of a previous study
[31]. Moreover, Proteus spp. was
resistant to all beta-lactams except imipenem, cefoxitin (a cephamycin) and
gentamicin (an aminoglycoside antibiotic). Furthermore, Escherichia coli were resistant to the majority of antibiotics tested,
except gentamicin and imipenem. Therefore, in our study, gentamicin and
imipenem were the most effective antibiotics against almost all bacteria from
the Enterobacteriaceae family, which is partially consistent with the results
of previous studies [32,33].
We
have found that Amikacin and Imipenem are the most effective antibiotic against
Gram-negative organisms, including Pseudomonas
aeruginosa. Differences in the results obtained in many studies show that
the patterns of microbial infection are not consistent in patients with DFU;
therefore, repeated evaluation of microbial characteristics and the antibiotic
sensitivity is necessary for the selection of appropriate antibiotics [6].
In
our study, fungal infection caused by Candida non-albicans, Candida albicans, Mucomycosis also
involve in creating an atmosphere, which cause DFU. Candida albicans is the main etiologic Candida species associated
with various type of disease including diabetes and related ulcers [34].
Several non-albicans Candida species like C.
glabrata, C. parapsilosis, C. tropicalis, C. krusei and C. auris,
etc., are more likely to be antifungal resistant and have the potential to
cause outbreaks of diseases [35]. Therefore, above findings highlighted that
Amikacin, Ciprofloxacin, Meropenem, Imipenem and Ceftriaxone were most
sensitive antibiotics in the cure of DFU caused by microorganisms.
A
common risk factor for the development of highly resistant bacteria is the
previous use of broad-spectrum antibiotics. In our study, all patients had
received antibiotic therapy prior to surgical debridement and this may explain
the higher rate of multidrug-resistant bacteria present in the diabetic foot
lesions in our study. Patients with DFU are usually hospitalized multiple times
and are often exposed to multiple courses of antibiotics [36], which may
influence antibiotic resistance. Therefore, the potential presence of such
resistant strains emphasizes the importance of obtaining optimal specimens from
diabetic foot infections for culture and sensitivity testing [36,37] as well as
the need to avoid excessive antibiotic therapy that promotes this resistance.
CONCLUSION
The
present study reports the high prevalence of multidrug-resistant pathogens in
diabetic foot ulcers. DFU were predominantly due to gram-negative bacteria,
such as Escherichia coli, Pseudomonas spp. and Klebsiella oxytoca. Many studies on the
bacteriology of DFU have reported results that vary and are often contradictory
[38,39]. In such cases, the application of molecular techniques may lead to
more accurate microbial characterizations and targeted antibiotic therapy.
Therefore, it is necessary to evaluate the different microorganisms infecting
the wound on a routine basis and to know the antibiotic susceptibility patterns
of the isolates from the infected wound in patients. This knowledge is crucial
for planning the treatment of these patients with the appropriate antibiotics,
reducing resistance patterns, and minimizing healthcare costs. We hope the data
presented in this article can assist the clinicians in determining the
multidrug-resistant pathogens in DFU.
DATA AVAILABILITY
All
the data created and used to support the findings of this study are included
within the article.
CONFLICT OF INTEREST
On
behalf of all authors, the corresponding author states that there are no
conflicts of interest.
FUNDING
No
funding was available.
ACKNOWLEDGEMENT
We
would like to thank the patient for participating in this study and for their
contribution to medical literature on this subject.
1.
Hu FB (2011) Globalization of diabetes: The role of
diet, lifestyle and genes. Diabetes Care 34: 1249-1257.
2.
Hobizal KB, Wukich DK (2012) Diabetic foot infections:
Current concept review. Diabet Foot Ankle 3: 10.
3.
Grennan D (2019) Diabetic foot ulcers. JAMA 321: 114.
4.
Wu SC, Driver VR, Wrobel JS, Armstrong DG (2007) Foot
ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk
Manag 3: 65-76.
5.
Sumpio BE (2012) Contemporary evaluation and management
of the diabetic foot. Scientifica (Cairo) 2012: 435487.
6.
Singh N, Armstrong DG, Lipsky BA (2005) Preventing foot
ulcers in patients with diabetes. JAMA 293: 217-228.
7.
Alexiadou K, Doupis J (2012) Management of diabetic
foot ulcers. Diabetes Ther 3: 4.
8.
Lipsky BA, Berendt AR, Deery HG, Embil JM, et al.
(2004) Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 39:
885-910.
9.
Viswanathan V, Thomas N, Tandon N, Asirvatham A,
Rajasekar S, et al. (2005) Profile of diabetic foot complications and its
associated complications - A multicentric study from India. J Assoc Phys India
53: 933-936.
10.
Ramachandran A (2005) Epidemiology of diabetes in India
- Three decades of research. J Assoc Phys India 53: 34-38.
11.
Jeffcoate WJ, Harding KG (2003) Diabetic foot ulcers.
Lancet (London, England) 361: 1545-1551.
12.
Rauwerda JA (2000) Foot debridement: Anatomic knowledge
is mandatory. Diabetes Metab Res Rev 16: 23-26.
13.
Dalla Paola L, Faglia E (2006) Treatment of diabetic
foot ulcer: An overview strategy for clinical approach. Curr Diabetes Rev 2:
431-447.
14.
Laing P (1994) Diabetic foot ulcers. Am J Surg 167:
31-36.
15.
Edmonds M (2006) Diabetic foot ulcers: Practical
treatment recommendations. Drugs 66: 913-929.
16.
Jorgensen JH, Ferraro MJ (2009) Antimicrobial
susceptibility testing: A review of general principles and contemporary
practices. Clin Infect Dis 49: 1749-1755.
17.
Gardner SE, Hillis SL, Frantz RA (2009) Clinical signs
of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs
11: 19-128.
18.
Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, et
al. (2014) Diabetic foot ulcers: Part I. Pathophysiology and prevention. J Am
Acad Dermatol 70: 1-18.
19.
Edmonds ME (1986) The diabetic foot: pathophysiology
and treatment. Clin Endocrinol Metab 15: 889-916.
20.
Pendsey SP (2010) Understanding diabetic foot. Int J
Diabetes Dev Ctries 30: 75-79.
21.
Lipsky BA, Pecoraro RE, Ahroni JH (1990) Foot
ulceration and infections in elderly diabetics. Clin Geriatr Med 6: 747-769.
22.
Lipsky BA (2004) International consensus group on d,
treating the infected diabetic f. A report from the international consensus on
diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev 20:
68-77.
23.
Sivanmaliappan TS, Sevanan M (2011) Antimicrobial
susceptibility patterns of Pseudomonas
aeruginosa from diabetes patients with foot ulcers. Int J Microbiol 2011:
605195.
24.
El-Tahawy AT (2000) Bacteriology of diabetic foot.
Saudi Med J 21: 344-347.
25.
Hobizal KB, Wukich DK (2012) Diabetic foot infections:
Current concept review. Diabetic Foot Ankle 3: 18409.
26.
Lipsky BA (2008) New developments in diagnosing and
treating diabetic foot infections. Diabetes Metab Res Rev 24: 66-71.
27.
Kandemir O, Akbay E, Sahin E, Milcan A, Gen R (2007)
Risk factors for infection of the diabetic foot with multi-antibiotic resistant
microorganisms. J Infect 54: 439-445.
28.
Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ,
et al. (2012) 2012 Infectious Diseases Society of America clinical practice
guideline for the diagnosis and treatment of diabetic foot infections. Clin
Infect Dis 54: 132-173.
29.
Gottrup F, Apelqvist J, Bjarnsholt T, Cooper R, Moore
Z, et al. (2013) EWMA document: Antimicrobials and non-healing wounds.
Evidence, controversies and suggestions. J Wound Care 22: 1-89.
30.
Banashankari G, Rudresh H, Harsha A (2012) Prevalence
of gram negative bacteria in diabetic foot - A clinico-microbiological study.
Al Ameen J Med Sci 5: 222-224.
31.
Anandi C, Alaguraja D, Natarajan V, Ramanathan M,
Subramaniam CS, et al. (2004) Bacteriology of diabetic foot lesions. Indian J
Med Microbiol 22: 175-178.
32.
Umadevi S, Kumar S, Joseph NM, Easow JM, Kandhakumari
G, et al. (2011) Microbiological study of diabetic foot infections. Indian J
Med Specialities 2.
33.
Guinea J (2014) Global trends in the distribution of
Candida species causing candidemia. Clin Microbiol Infect 20: 5-10.
34.
Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A (2016)
First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control
5: 35.
35.
Gales AC, Reis AO, Jones RN (2001) Contemporary
assessment of antimicrobial susceptibility testing methods for polymyxin B and
colistin: Review of available interpretative criteria and quality control
guidelines. J Clin Microbiol 39: 183-190.
36.
Gales AC, Jones RN, Sader HS (2006) Global assessment
of the antimicrobial activity of polymyxin B against 54 731 clinical isolates
of gram-negative bacilli: Report from the SENTRY antimicrobial surveillance
programme (2001-2004). Clin Microb Infect 12: 315-321.
37.
Citron DM, Goldstein EJ, Merriam CV, Lipsky BA,
Abramson MA (2007) Bacteriology of moderate-to-severe diabetic foot infections
and in vitro activity of antimicrobial agents. J Clin Microbiol 45: 2819-2828.
38.
Abdulrazak A, Bitar ZI, Al-Shamali AA, Mobasher LA
(2005) Bacteriological study of diabetic foot infections. J Diabetes
Complications 19: 138-141.
39.
Gadepalli R, Dhawan B, Sreenivas V, Kapil A, Ammini AC,
et al. (2006) A clinico-microbiological study of diabetic foot ulcers in an
Indian tertiary care hospital. Diabetes Care 29: 1727-1732.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Astronomy and Space Research
- Journal of Womens Health and Safety Research (ISSN:2577-1388)